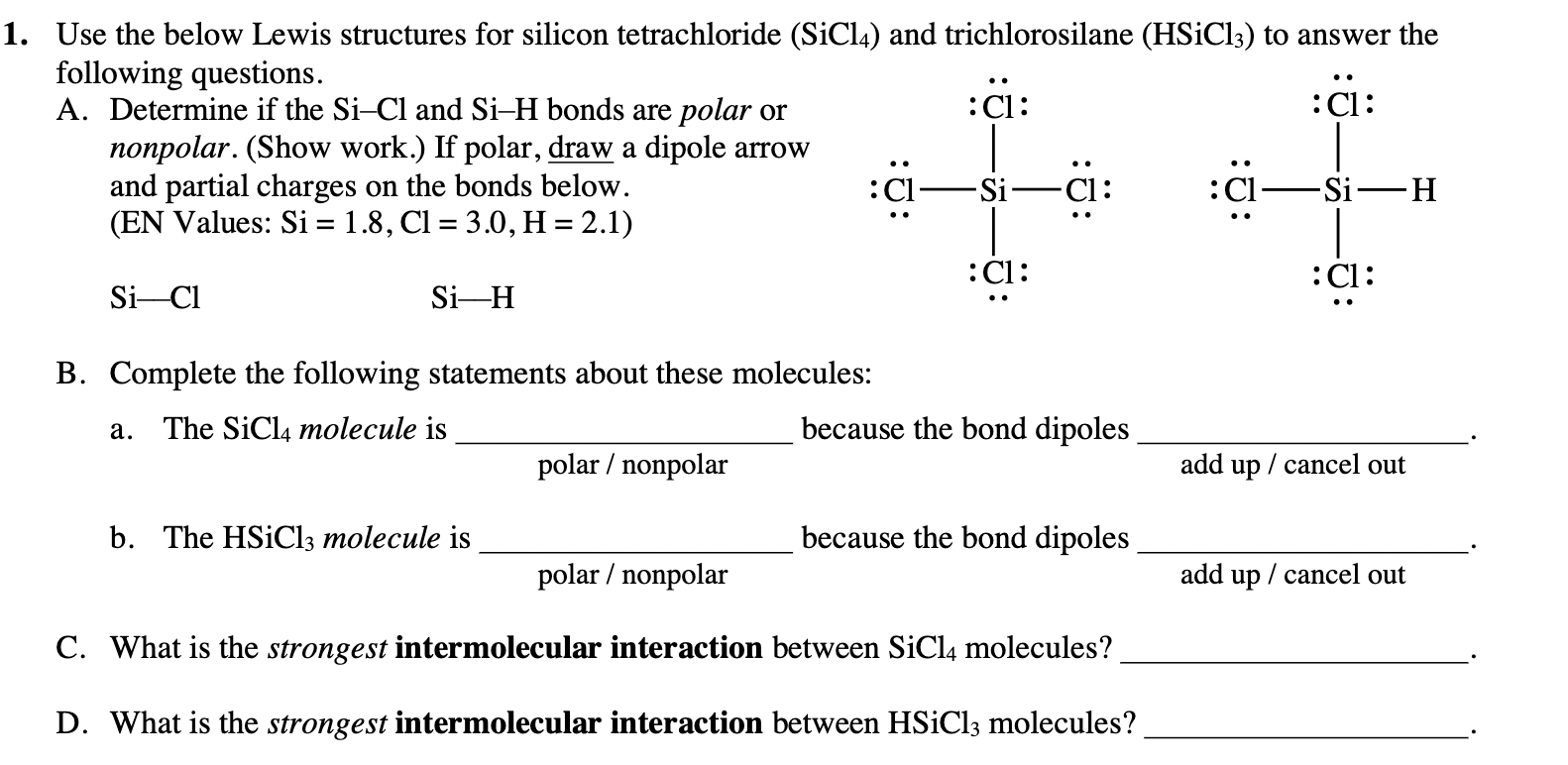
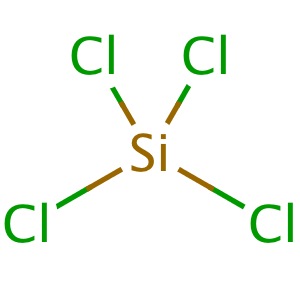
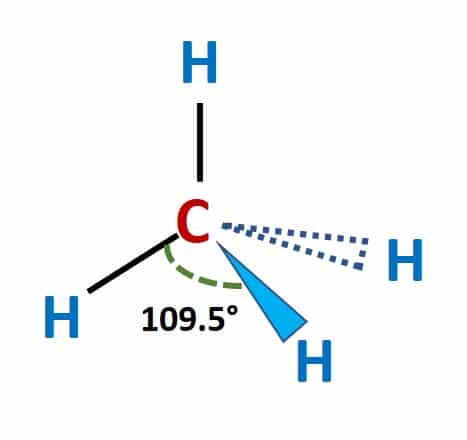
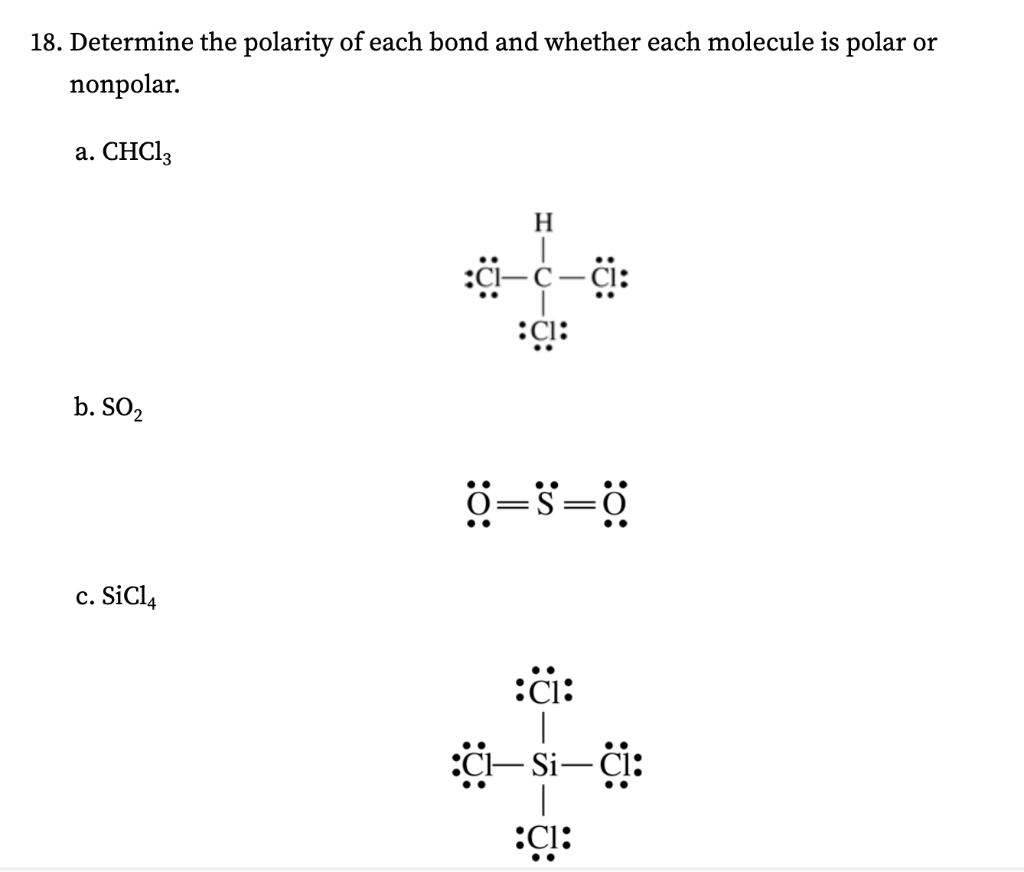
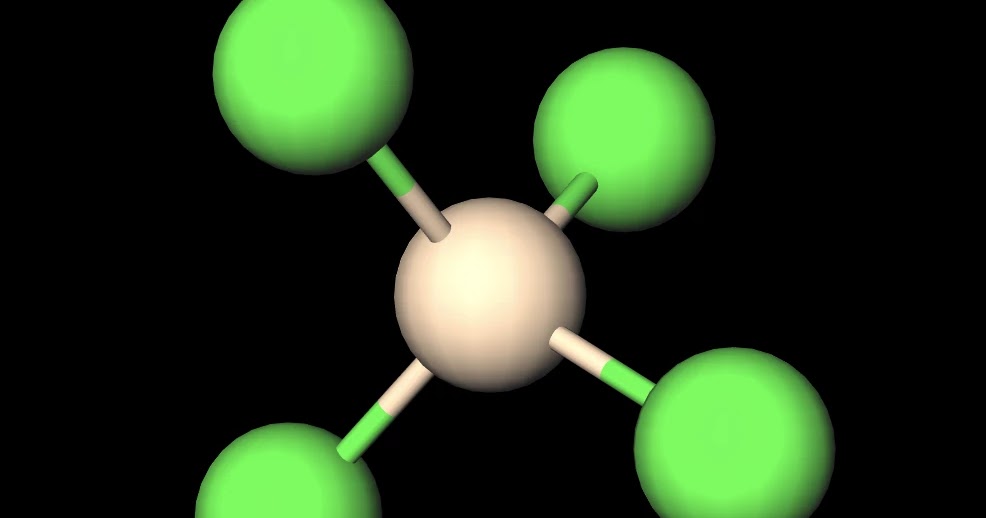
Is SiCl4 Polar or Non-polar? (Silicon Tetrachloride) | Is SiCl4 Polar or Non-polar? (Silicon Tetrachloride) We know the concept of polarity is a bit tricky and one might need a little practice
![The \\[SiC{l_4}\\] molecule is nonpolar and chlorine is more electronegative than silicon. From this information alone it can be deduced that:1. \\[Si-Cl\\] bond is nonpolar2. \\[SiC{l_4}\\] molecule is planar3. \\[SiC{l_4}\\] molecule is The \\[SiC{l_4}\\] molecule is nonpolar and chlorine is more electronegative than silicon. From this information alone it can be deduced that:1. \\[Si-Cl\\] bond is nonpolar2. \\[SiC{l_4}\\] molecule is planar3. \\[SiC{l_4}\\] molecule is](https://www.vedantu.com/question-sets/cff3a96a-22a9-491b-b4c7-e2a25d03a8828875529946816540776.png)
The \\[SiC{l_4}\\] molecule is nonpolar and chlorine is more electronegative than silicon. From this information alone it can be deduced that:1. \\[Si-Cl\\] bond is nonpolar2. \\[SiC{l_4}\\] molecule is planar3. \\[SiC{l_4}\\] molecule is

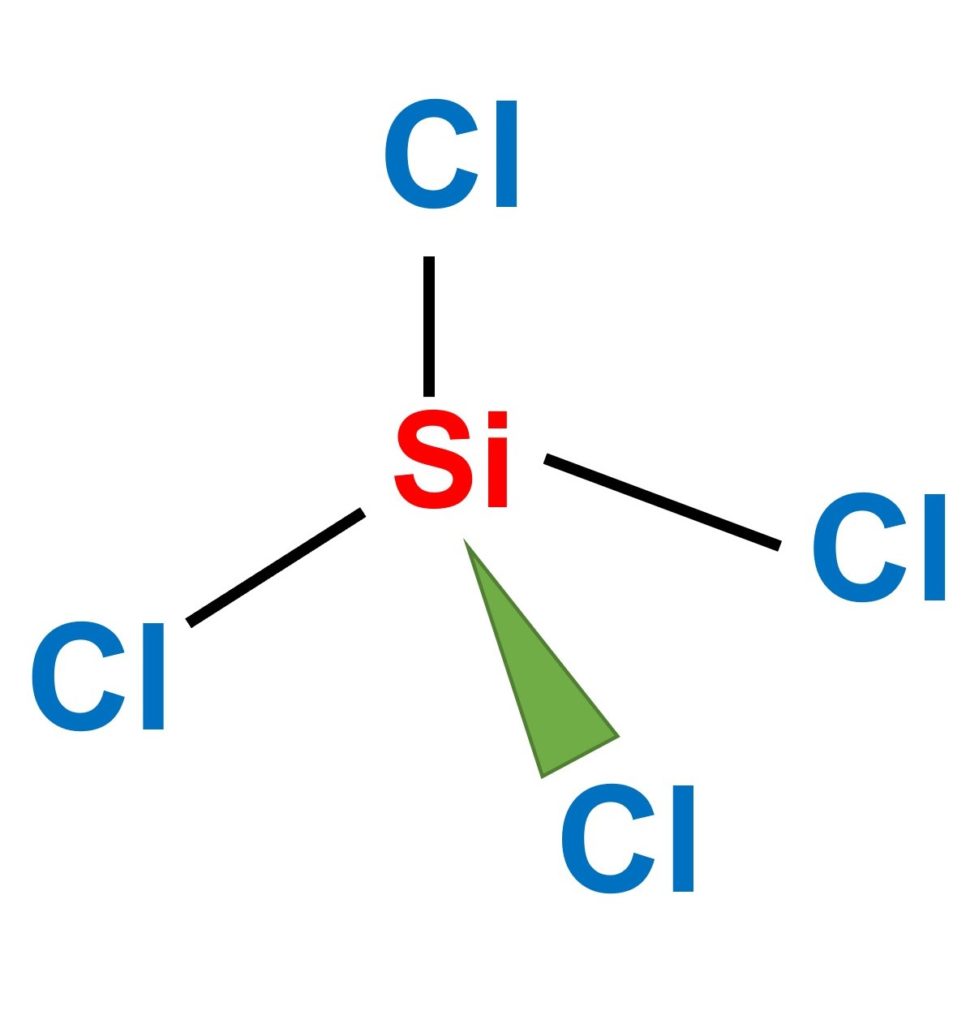
SiCl4 lewis structure, molecular geometry, hybridization, polar or nonpolar | Molecular geometry, Molecular shapes, Molecular